Abstract
Introduction: Subcutaneous (SC) administration offers several potential advantages for patients (pts) and healthcare providers, including shorter administration times, reduced administration volume, and fewer infusion-related reactions. Daratumumab (DARA), a human IgGκ anti-CD38 monoclonal antibody, has direct on-tumor and immunomodulatory effects. DARA is approved for intravenous (IV) administration as monotherapy for RRMM and in combination with standard of care regimens for RR and newly diagnosed MM. Previous analyses demonstrated that maximum DARA trough concentration (Ctrough), which occurs at the end of weekly dosing, was highly correlated with efficacy in MM (Xu XS, et al. Clin Pharmacol Ther 2017. 101[6]:721-724), but no relationship was observed between DARA concentrations and adverse events. SC administration of DARA in combination with recombinant human hyaluronidase enzyme PH20 (rHuPH20; ENHANZE® drug delivery technology, Halozyme, Inc.) is being evaluated in an open-label, phase 1b trial (PAVO) to identify a fixed SC dose which is safe and achieves a similar or greater maximum Ctrough as compared with the 16 mg/kg IV dose. Here, we present the clinical pharmacology of SC DARA, including the development of a combined IV-SC population PK (PPK) model.
Methods: In PAVO, eligible RRMM pts received ≥2 prior lines of therapy. In Part 1, 2 sequential cohorts received fixed dose regimens of a mix and deliver SC formulation of DARA and rHuPH20 (DARA MD) at 1,200 mg (n = 8; 60 mL) and 1,800 mg (n = 45; 90 mL) doses in 20 and 30 minutes, respectively, on the same schedule as the approved DARA monotherapy IV regimen (QW for Cycles 1-2, Q2W in Cycles 3-6, and Q4W thereafter; 4-week cycles). A dose that was safe and provided similar or greater Ctrough values on Cycle 3 Day 1 (C3D1) compared with the 16 mg/kg IV dose was selected for Part 2. In Part 2, a concentrated co-formulation of DARA (1,800 mg) and rHuPH20 (30,000 U; in 15 mL) in a single, pre-mixed vial (DARA SC) was administered over 3-5 minutes by manual SC injection. Co-primary endpoints were safety and Ctrough at the end of QW dosing. Serial PK sampling was performed after the first and last weekly dose; sparse PK and immunogenicity was collected at additional timepoints. A mixed-effects 2-compartment PK model based on Michaelis-Menten approximation of target-mediated drug disposition was developed, and PK data used in this PPK analysis were from Part 1 of PAVO (n = 53) and from GEN501/MMY2002 (IV DARA monotherapy; n = 223) studies. Model-based simulations were conducted to facilitate dose selection.
Results: In Part 1, the mean C3D1 Ctrough following 1,800 mg DARA MD was similar or higher than that observed for 16 mg/kg IV (Table). The PPK model predicted approximately 88% of pts receiving DARA MD 1,800 mg would achieve the effective concentration of 274 μg/mL vs 73% of pts receiving DARA MD 1,200 mg and 80% of pts receiving 16 mg/kg IV. Simulated PK profiles indicated that both DARA MD doses result in smaller peak-to-trough fluctuation compared to IV dosing, and that 1,800 mg provides higher Ctrough throughout the dosing period without increasing Cmax compared to IV dosing (Usmani SZ, et al. ASH 2016. Abs. 1149). Across a range of simulated body weights, exposure following DARA MD 1,800 mg was similar or slightly higher than for 16 mg/kg IV.
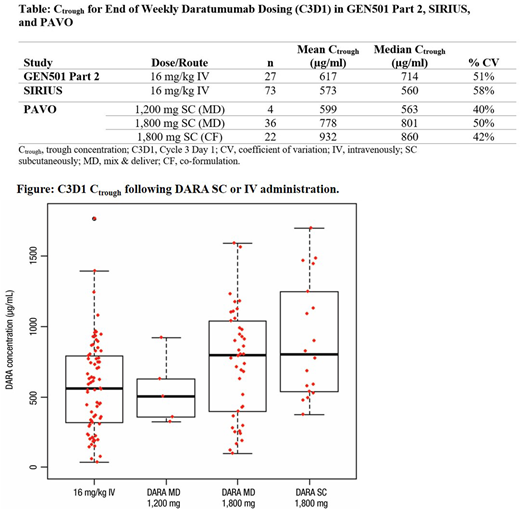
Based on these data, the 1,800 mg dose was selected for further evaluation in Part 2. The primary PK endpoint of mean C3D1 Ctrough was slightly higher for DARA SC 1,800 mg than the DARA MD 1,800 mg cohort and 16 mg/kg IV dose (Table). The range of Ctrough observations for the SC cohorts were within the range observed with 16 mg/kg IV (Figure). The observed Cmax of DARA SC 1,800 mg after the first and last weekly dose was within the range of that for 16 mg/kg IV; inter-pt variability for SC administered DARA was also similar across all cohorts.
One pt (1%) was positive for DARA anti-drug antibodies and 13% of pts were positive for anti-rHuPH20 antibodies in PAVO; all had no apparent clinical complications.
Conclusions: The fixed DARA SC 1,800 mg dose provided a similar or higher C3D1 Ctrough when compared to historical data with 16 mg/kg IV. A combined IV-SC PPK model using a concentration- and time-dependent clearance of DARA described the PAVO data well. Immunogenicity of DARA and rHuPH20 was similar to previous experience. These data validated the dose selection of 1,800 mg for ongoing phase 3 clinical trials of DARA SC in MM, smoldering MM, and amyloidosis.
Clemens:Janssen Research & Development, LLC: Employment. Xu:Janssen Research & Development, LLC: Employment. Luo:Janssen Research & Development, LLC: Employment. Chari:Array Biopharma: Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy; Pharmacyclics: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; The Binding Site: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Usmani:Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics,Sanofi, Seattle Genetics, Takeda: Research Funding; Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Janssen, Seattle Genetics: Consultancy. Mateos:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. van de Donk:Janssen Pharmceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Research Funding; Novartis: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding. Kaufman:Abbvie: Consultancy; Roche: Consultancy; Janssen: Consultancy; Karyopharm: Other: data monitoring committee; BMS: Consultancy. Moreau:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oriol:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Plesner:Janssen: Consultancy; Celgene: Other: Independent Response Assessment Comittee. San-Miguel:Celgene: Honoraria; Amgen: Honoraria; BMS: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; Roche: Honoraria; Janssen: Honoraria. Sun:Janssen Research & Development, LLC: Employment. Farnsworth:Janssen Research & Development: Employment. Masterson:Janssen Research & Development, LLC: Employment. Hellemans:Janssen Research & Development: Employment. Qi:Janssen Research & Development, LLC: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal